A-level Chemistry
A-level Chemistry is a rigorous, challenging and extremely rewarding course that develops students’ scientific skills and knowledge. Learn to see the world differently with Chemistry as you discover how different elements of science combine. This course will equip you with unique skills that will serve you well in further education or in employment.
Choose your learning path
Unlimited Student Support™
Receive one-on-one personalised guidance and support from our expert personal tutors.
With our Unlimited Student Support™, you can get as much or as little help as you want throughout your course. You'll benefit from one-to-one attention from a qualified and experienced tutor, tailored to your learning requirements.
Whether you have questions about assignments, need assistance with a complex topic or just need a bit of motivation, your personal tutor and our learning support team are here to support your learning journey at every step of your learning journey.



Homeschool Online Courses
Oxbridge courses are designed with homeschooling in mind. Whether you're a parent guiding your child's education or an independent learner, our flexible online format fits around your schedule. With unlimited student support, structured cirriculum lessons, and exam preparation tailored for home learners, we make homeschooling simple, effective and fully supported.

MyOxbridge™
Take control of your learning experience with MyOxbridge™, our award-winning online learning platform.
You can access your course materials, submit assignments, view grades, track progress, and get support anytime, anywhere, via mobile, computer, or tablet. We also include many common accessibility features as standard.
Our online students achieve 20% higher grades than they would at a traditional college. With MyOxbridge, you can exceed your learning goals, on your terms.
award-winning online platform. Access course materials anytime, anywhere; on mobile, laptop, or tablet. Submit assignments, view grades, and track progress with engaging content and offline access. Achieve your goals flexibly!
Prefer old-school?
Download our prospectus…
Don't worry; we're not precious about everything being online. You can also download an old-school prospectus, and many of our courses can be sent to you in printed format. Our prospectus tells you all about learning with Oxbridge.
Request free prospectus
Student perks with Oxbridge

Unlock exclusive student discounts on popular brands with Student Beans. After enrolling at Oxbridge, register to access free codes and deals on shopping and food.
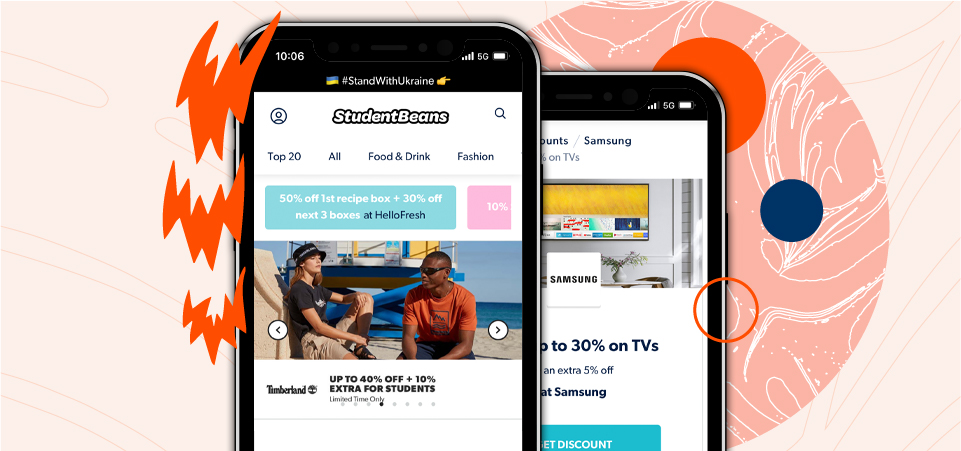

The #1 student discount card and app. Access deals on food, essentials, tech, travel, fashion, and more. Get exclusive coupons and discounts in-store and online, all from your pocket.

Need more information? Chat with a Learning Adviser
We're happy to help and talk you through anything about this course that you want more information about. You can contact us or use the most popular methods below.
Our live chat is supported by incredibly friendly human beings.